Gainesville Biomass Project
3:21 AM
Two rulings announced on Dec. 7 moved the Gainesville Renewable Energy Center closer to a construction start.
The Florida Power Plant Siting Board, composed of the Florida governor and the independently elected members of the Cabinet, unanimously approved the site application for the proposed biomass power plant in Gainesville, Fla. This siting board approval is the culmination of the permitting and regulatory activities designed to ensure that the project is in the best interests of the citizens of Florida. In announcing the approval, Gov. Charlie Crist said, “I think this can be a great breakthrough and I think it is the right thing to do. The groups that have supported this, I have great trust and confidence in them.”
Also on Dec. 7, a Florida administrative law judge issued his second ruling in favor of the proposed biomass plant. Judge Robert E. Meale’s recommended orders for the plant’s air construction permit rejected claims made by petitioners who oppose the plant. He found instead that the facility will comply with all applicable environmental regulations and will not cause adverse air emissions impacts or adverse impacts to wildlife species or their habitat. The air construction permit is expected to be finalized by the Florida Department of Environmental Protection in the next few weeks.
On Nov. 1, in a separate but related process for the site certification application, Judge Meale issued an order recommending that a site certification be granted for the Gainesville Renewable Energy Center despite the claims of one intervenor. In that order, Judge Meale stated, “Instead of undermining sound silvicultural practices, the new market for biomass materials will enhance the viability of forestry resources and thus serve regional environmental needs.”
The 100-megawatt biomass project will be owned and operated by American Renewables,
LLC. Gainesville Regional Utilities, the municipally-owned utility that serves the Gainesville community, has a 30-year power purchase agreement to buy all power generated. GRU chose to move forward with GREC after undergoing a seven-year process to review options to responsibly and cost-effectively meet its future generation needs while helping Gainesville achieve its carbon reduction goals. GREC, which will be fueled by wood waste, will meet GRU’s need for improved reliability, increased fuel diversity and long-term cost savings for customers.
Almost two-thirds of the energy GRU currently produces is fueled by coal, and 25 percent comes from natural gas.
The three national bond rating agencies that recently awarded the utility “Double A” ratings cited a lack of fuel diversity as one of the challenges facing GRU. Adding biomass to the fuel supply will help the utility maintain its high bond ratings, which in the past six years have saved customers more than $67 million.
“The siting board’s decision and the Judge’s order confirm that GREC will be designed and operated in an environmentally responsible manner,” said Josh Levine, project manager for American Renewables. “We look forward to quickly beginning construction.” Levine added. “It is critical that the project move forward without delay so the citizens of Gainesville and the region can reap the significant economic benefits the plant will bring, including more than 700 direct and indirect permanent jobs throughout the region, as well as the benefit of nearly $200 million in reduced rates over 30 years if the project remains on schedule and is eligible for federal stimulus dollars.”
The plant has been approved and endorsed by a wide variety of government agencies, nonprofits and other organizations from the environmental, business, forestry and other communities, including: the Florida Wildlife Federation, Southern Alliance for Clean Energy, Florida Forestry Association, Florida Farm Bureau Federation, Florida Municipal Electric Association, Gainesville Area Chamber of Commerce, FloridaWorks, Forest Landowners Association, Alachua County Legislative Delegation, Gainesville City Commissioners, North Central Florida Renewable Resource Conservation & Development Council, Florida Department of Agriculture & Consumer Services, Florida Public Service Commission, Florida Department of Environmental Protection, Florida Department of Health, Florida Department of Community Affairs, Florida Department of State, Florida Department of Transportation, North Central Florida Regional Planning Council, Suwannee River Water Management District, City of Gainesville, Alachua County and the U.S. Corps of Engineers.
Source:http://biomassmagazine.com/articles/5162/gainesville-biomass-project-clears-regulatory-hurdles/
Malavalli Biomass Power Plant
3:19 AM
| Project Type: | Biomass Combustion |
| Location: | Kirugaval village, Malavalli Taluka, Mandya District, Karnataka, India |
| Quantity: | 145,000 tonnes CO2e total |
| Accounting period: | 7 years |
| Operator: | Malavalli Power Plant Pvt Ltd. 3rd Floor Gupta Towers 50/1 Residency Road 1st Cross Bangalore Karnataka 560025 |
| Certification: | Clean Development Mechanism Gold Standard |
| Supported by: | Climat Mundi (France) Less (Canada) myclimate (Switzerland) Planetair (Canada) PURE (UK) South Pole Carbon Asset Managment Ltd. (Switzerland) Sustainable Travel International (USA - WA) ZeroGHG (Canada) |
Anaerobic Digestion for Power Generation
4:12 AM
It is widely used as part of the process to treat wastewater. As part of an integrated waste management system, anaerobic digestion reduces the emission of landfill gas into the atmosphere.
 Anaerobic digestion is widely used as a renewable energy source because the process produces a methane and carbon dioxide rich biogas suitable for energy production, helping to replace fossil fuels. The nutrient-rich digestate which is also produced can be used as fertilizer.
Anaerobic digestion is widely used as a renewable energy source because the process produces a methane and carbon dioxide rich biogas suitable for energy production, helping to replace fossil fuels. The nutrient-rich digestate which is also produced can be used as fertilizer.The Anaerobic digestion process begins with bacterial hydrolysis of the input materials in order to break down insoluble organic polymers such as carbohydrates and make them available for other bacteria. Acidogenic bacteria then convert the sugars and amino acids into carbon dioxide, hydrogen, ammonia, and organic acids. Acetogenic bacteria then convert these resulting organic acids into acetic acid, along with additional ammonia, hydrogen, and carbon dioxide. Finally, methanogens convert these products to methane and carbon dioxide.
The technical expertise required to maintain industrial scale anaerobic digesters coupled with high capital costs and low process efficiencies had limited the level of its industrial application as a waste treatment technology. Anaerobic digestion facilities have, however, been recognized by the United Nations Development Programme as one of the most useful decentralized sources of energy supply, as they are less capital intensive than large power plants.
Biogas from sewage works is sometimes used to run a gas engine to produce electrical power; some or all of which can be used to run the sewage works. Some waste heat from the engine is then used to heat the digester. It turns out that the waste heat is generally enough to heat the digester to the required temperatures. The power potential from sewage works is limited – in the UK there are about 80 MW total of such generation, with potential to increase to 150 MW, which is insignificant compared to the average power demand in the UK of about 35,000 MW. The scope for biogas generation from non-sewage waste biological matter – energy crops, food waste, abattoir waste etc. is much higher, estimated to be capable of about 3,000 MW. Farm biogas plants using animal waste and energy crops are expected to contribute to reducing CO2 emissions and strengthen the grid while providing UK farmers with additional revenues.
Some countries offer incentives in the form of, for example, Feed-in Tariffs for feeding electricity onto the power grid in order to subsidize green energy production.
Biomass Pyrolysis
5:19 AM
Pyrolysis is a thermochemical decomposition of organic material at elevated temperatures in the absence of oxygen. Pyrolysis typically occurs under pressure and at operating temperatures above 430 °C (800 °F). In practice it is not possible to achieve a completely oxygen-free atmosphere. Because some oxygen is present in any pyrolysis system, a small amount of oxidation occurs. The word is coined from the Greek-derived elements pyr "fire" and lysis "loosening".
Pyrolysis is a special case of thermolysis, and is most commonly used for organic materials, being then one of the processes involved in charring. The pyrolysis of wood, which starts at 200–300 °C (390–570 °F), occurs for example in fires or when vegetation comes into contact with lava in volcanic eruptions. In general, pyrolysis of organic substances produces gas and liquid products and leaves a solid residue richer in carbon content. Extreme pyrolysis, which leaves mostly carbon as the residue, is called carbonization.
This chemical process is heavily used in the chemical industry, for example, to produce charcoal, activated carbon, methanol and other chemicals from wood, to convert ethylene dichloride into vinyl chloride to make PVC, to produce coke from coal, to convert biomass into syngas, to turn waste into safely disposable substances, and for transforming medium-weight hydrocarbons from oil into lighter ones like gasoline. These specialized uses of pyrolysis may be called various names, such as dry distillation, destructive distillation, or cracking.
Pyrolysis also plays an important role in several cooking procedures, such as baking, frying, grilling, and caramelizing. And it is a tool of chemical analysis, for example in mass spectrometry and in carbon-14 dating. Indeed, many important chemical substances, such as phosphorus and sulphuric acid, were first obtained by this process. Pyrolysis has been assumed to take place during catagenesis, the conversion of buried organic matter to fossil fuels. It is also the basis of pyrography. In their embalming process, the ancient Egyptians used a mixture of substances, including methanol, which they obtained from the pyrolysis of wood.
Pyrolysis differs from other high-temperature processes like combustion and hydrolysis in that it does not involve reactions with oxygen, water, or any other reagents. However, the term has also been applied to the decomposition of organic material in the presence of superheated water or steam (hydrous pyrolysis), for example in the steam cracking of oil.
Occurrence and uses
Fire
Pyrolysis is usually the first chemical reaction that occurs in the burning of many solid organic fuels, like wood, cloth, and paper, and also of some kinds of plastic. In a wood fire, the visible flames are not due to combustion of the wood itself, but rather of the gases released by its pyrolysis; whereas the flame-less burning of embers is the combustion of the solid residue (charcoal) left behind by it. Thus, the pyrolysis of common materials like wood, plastic, and clothing is extremely important for fire safety and fire-fighting.
Cooking
Pyrolysis occurs whenever food is exposed to high enough temperatures in a dry environment, such as roasting, baking, toasting, grilling, etc.. It is the chemical process responsible for the formation of the golden-brown crust in foods prepared by those methods.
In normal cooking, the main food components that suffer pyrolysis are carbohydrates (including sugars, starch, and fibre) and proteins. Pyrolysis of fats requires a much higher temperature, and since it produces toxic and flammable products (such as acrolein), it is generally avoided in normal cooking. It may occur, however, when barbecuing fatty meats over hot coals.
Even though cooking is normally carried out in air, the temperatures and environmental conditions are such that there is little or no combustion of the original substances or their decomposition products. In particular, the pyrolysis of proteins and carbohydrates begins at temperatures much lower than the ignition temperature of the solid residue, and the volatile subproducts are too diluted in air to ignite. (In flambé dishes, the flame is due mostly to combustion of the alcohol, while the crust is formed by pyrolysis as in baking.)
Pyrolysis of carbohydrates and proteins require temperatures substantially higher than 100 °C (212 °F), so pyrolysis does not occur as long as free water is present, e.g. in boiling food — not even in a pressure cooker. When heated in the presence of water, carbohydrates and proteins suffer gradual hydrolysis rather than pyrolysis. Indeed, for most foods, pyrolysis is usually confined to the outer layers of food, and only begins after those layers have dried out.
Food pyrolysis temperatures are however lower than the boiling point of lipids, so pyrolysis occurs when frying in vegetable oil or suet, or basting meat in its own fat.
Pyrolysis also plays an essential role in the production of barley tea, coffee, and roasted nuts such as peanuts and almonds. As these consist mostly of dry materials, the process of pyrolysis is not limited to the outermost layers but extends throughout the materials. In all these cases, pyrolysis creates or releases many of the substances that contribute to the flavor, color, and biological properties of the final product. It may also destroy some substances that are toxic, unpleasant in taste, or those that may contribute to spoilage.
Controlled pyrolysis of sugars starting at 170 °C (338 °F) produces caramel, a beige to brown water-soluble product which is widely used in confectionery and (in the form of caramel coloring) as a coloring agent for soft drinks and other industrialized food products.
Solid residue from the pyrolysis of spilled and splattered food creates the brown-black encrustation often seen on cooking vessels, stove tops, and the interior surfaces of ovens.
Charcoal
Pyrolysis has been used since ancient times for turning wood into charcoal in an industrial scale. Besides wood, the process can also use sawdust and other wood waste products.
Charcoal is obtained by heating wood until its complete pyrolysis (carbonization) occurs, leaving only carbon and inorganic ash. In many parts of the world, charcoal is still produced semi-industrially, by burning a pile of wood that has been mostly covered with mud or bricks. The heat generated by burning part of the wood and the volatile byproducts pyrolyzes the rest of the pile. The limited supply of oxygen prevents the charcoal from burning too. A more modern alternative is to heat the wood in an airtight metal vessel, which is much less polluting and allows the volatile products to be condensed.
The original vascular structure of the wood and the pores created by escaping gases combine to produce a light and porous material. By starting with dense wood-like material, such as nutshells or peach stones, one obtains a form of charcoal with particularly fine pores (and hence a much larger pore surface area), called activated carbon, which is used as an adsorbent for a wide range of chemical substances.
Biochar
Residues of incomplete organic pyrolysis, e.g. from cooking fires, are thought to be the key component of the terra preta soils associated with ancient indigenous communities of the Amazon basin. Terra preta is much sought by local farmers for its superior fertility compared to the natural red soil of the region. Efforts are underway to recreate these soils through biochar, the solid residue of pyrolysis of various materials, mostly organic waste.
Biochar improves the soil texture and ecology, increasing its ability to retain fertilizers and release them slowly. It naturally contains many of the micronutrients needed by plants, such as selenium. It is also safer than other "natural" fertilizers such as manure or sewage since it has been disinfected at high temperature, and since it releases its nutrients at a slow rate, it greatly reduces the risk of water table contamination.
Biochar is also being considered for carbon sequestration, with the aim of mitigation of global warming.
Coke
Pyrolysis is used on a massive scale to turn coal into coke for metallurgy, especially steelmaking. Coke can also be produced from the solid residue left from petroleum refining.
Those starting materials typically contain hydrogen, nitrogen or oxygen atoms combined with carbon into molecules of medium to high molecular weight. The coke-making or "coking" process consists in heating the material in closed vessels to very high temperatures (up to 2,000 °C or 3,600 °F), so that those molecules are broken down into lighter volatile substances, which leave the vessel, and a porous but hard residue that is mostly carbon and inorganic ash. The amount of volatiles varies with the source material, but is typically 25-30% of it by weight.
Carbon fiber
Carbon fibers are filaments of carbon that can be used to make very strong yarns and textiles. Carbon fiber items are often produced by spinning and weaving the desired item from fibers of a suitable polymer, and then pyrolyzing the material at a high temperature (from 1,500–3,000 °C or 2,730–5,430 °F).
The first carbon fibers were made from rayon, but polyacrylonitrile has become the most common starting material.
For their first workable electric lamps, Joseph Wilson Swan and Thomas Edison used carbon filaments made by pyrolysis of cotton yarns and bamboo splinters, respectively.
Biofuel
Pyrolysis is the basis of several methods that are being developed for producing fuel from biomass, which may include either crops grown for the purpose or biological waste products from other industries.
Although synthetic diesel fuel cannot yet be produced directly by pyrolysis of organic materials, there is a way to produce similar liquid ("bio-oil") that can be used as a fuel, after the removal of valuable bio-chemicals that can be used as food additives or pharmaceuticals.[8] Higher efficiency is achieved by the so-called flash pyrolysis where finely divided feedstock is quickly heated to between 350 and 500 °C (660 and 930 °F) for less than 2 seconds.
Fuel bio-oil resembling light crude oil can also be produced by hydrous pyrolysis from many kinds of feedstock, including waste from pig and turkey farming, by a process called thermal depolymerization (which may however include other reactions besides pyrolysis).
Plastic waste disposal
Anhydrous pyrolysis can also be used to produce liquid fuel similar to diesel from plastic waste.
Biomass Pyrolysis Processes
In many industrial applications, the process is done under pressure and at operating temperatures above 430 °C (806 °F). For agricultural waste, for example, typical temperatures are 450 to 550 °C (840 to 1,000 °F).
Vacuum pyrolysis
In vacuum pyrolysis, organic material is heated in a vacuum in order to decrease boiling point and avoid adverse chemical reactions. It is used in organic chemistry as a synthetic tool. In flash vacuum thermolysis or FVT, the residence time of the substrate at the working temperature is limited as much as possible, again in order to minimize secondary reactions.
Processes for biomass pyrolysis
Since pyrolysis is endothermic, various methods have been proposed to provide heat to the reacting biomass particles:
-
- Partial combustion of the biomass products through air injection. This results in poor-quality products.
- Direct heat transfer with a hot gas, ideally product gas that is reheated and recycled. The problem is to provide enough heat with reasonable gas flow-rates.
- Indirect heat transfer with exchange surfaces (wall, tubes). It is difficult to achieve good heat transfer on both sides of the heat exchange surface.
- Direct heat transfer with circulating solids: Solids transfer heat between a burner and a pyrolysis reactor. This is an effective but complex technology.
For flash pyrolysis the biomass must be ground into fine particles and the insulating char layer that forms at the surface of the reacting particles must be continuously removed. The following technologies have been proposed for biomass pyrolysis:
-
- Fixed beds were used for the traditional production of charcoal. Poor, slow heat transfer resulted in very low liquid yields.
- Augers: This technology is adapted from a Lurgi process for coal gasification. Hot sand and biomass particles are fed at one end of a screw. The screw mixes the sand and biomass and conveys them along. It provides a good control of the biomass residence time. It does not dilute the pyrolysis products with a carrier or fluidizing gas. However, sand must be reheated in a separate vessel, and mechanical reliability is a concern. There is no large-scale commercial implementation.
- Ablative processes: Biomass particles are moved at high speed against a hot metal surface. Ablation of any char forming at the particles surface maintains a high rate of heat transfer. This can be achieved by using a metal surface spinning at high speed within a bed of biomass particles, which may present mechanical reliability problems but prevents any dilution of the products. As an alternative, the particles may be suspended in a carrier gas and introduced at high speed through a cyclone whose wall is heated; the products are diluted with the carrier gas. A problem shared with all ablative processes is that scale-up is made difficult since the ratio of the wall surface to the reactor volume decreases as the reactor size is increased. There is no large-scale commercial implementation.
- Rotating cone: Pre-heated hot sand and biomass particles are introduced into a rotating cone. Due to the rotation of the cone, the mixture of sand and biomass is transported across the cone surface by centrifugal force. Like other shallow transported-bed reactors relatively fine particles are required to obtain a good liquid yield. There is no large scale commercial implementation.
- Fluidized beds: Biomass particles are introduced into a bed of hot sand fluidized by a gas, which is usually a recirculated product gas. High heat transfer rates from fluidized sand result in rapid heating of biomass particles. There is some ablation by attrition with the sand particles, but it is not as effective as in the ablative processes. Heat is usually provided by heat exchanger tubes through which hot combustion gas flows. There is some dilution of the products, which makes it more difficult to condense and then remove the bio-oil mist from the gas exiting the condensers. This process has been scaled up by companies such as Dynamotive and Agri-Therm. The main challenges are in improving the quality and consistency of the bio-oil.
- Circulating fluidized beds: Biomass particles are introduced into a circulating fluidized bed of hot sand. Gas, sand and biomass particles move together, with the transport gas usually being a recirculated product gas, although it may also be a combustion gas. High heat transfer rates from sand ensure rapid heating of biomass particles and ablation is stronger than with regular fluidized beds. A fast separator separates the product gases and vapors from the sand and char particles. The sand particles are reheated in fluidized burner vessel and recycled to the reactor. Although this process can be easily scaled up, it is rather complex and the products are much diluted, which greatly complicates the recovery of the liquid products.
Industrial sources
Many sources of organic matter can be used as feedstock for pyrolosis. Suitable plant material includes: greenwaste, sawdust, waste wood, woody weeds; and agricultural sources including: nut shells, straw, cotton trash, rice hulls, switch grass; and poultry litter, dairy manure and potentially other manures. Pyrolysis is used as a form of thermal treatment to reduce waste volumes of domestic refuse. Some industrial byproducts are also suitable feedstock including paper sludge and distillers grain.
There is also the possibility of integrating with other processes such as mechanical biological treatment and anaerobic digestion.
Industrial products
- syngas (flammable mixture of carbon monoxide and hydrogen): can be produced in sufficient quantities to both provide the energy needed for pyrolysis and some excess production
- solid char that can either be burned for energy or recycled as a fertilizer (biochar).
Pyrolysis Fire protection
Destructive fires in buildings will often burn with limited oxygen supply, resulting in pyrolysis reactions. Thus, pyrolysis reaction mechanisms and the pyrolysis properties of materials are important in fire protection engineering for passive fire protection. Pyrolytic carbon is also important to fire investigators as a tool for discovering origin and cause of fires.
Torrefaction of Biomass
1:20 PM

Torrefaction of biomass can be described as a mild form of pyrolysis at temperatures typically ranging between 200-320 °C. During torrefaction the biomass properties are changed to obtain a much better fuel quality for combustion and gasification applications. Torrefaction combined with densification leads to a very energy dense fuel carrier of 20-25 GJ/ton.
Biomass can be an important energy source to create a more sustainable society. However, nature has created a large diversity of biomass with varying specifications. In order to create highly efficient biomass-to-energy chains, torrefaction of biomass in combination with densification (pelletisation/briquetting), is a promising step to overcome logistic economics in large scale green energy solutions.
Process of Torrefaction
Torrefaction is a thermo chemical treatment of biomass at 200 to 320 °C. It is carried out under atmospheric conditions and in the absence of oxygen. During the process, the water contained in the biomass as well as superfluous volatiles are removed, and the biopolymers (cellulose, hemicellulose and lignin) partly decompose giving off various types of volatiles. The final product is the remaining solid, dry, blackened material which is referred to as “torrefied biomass” or “bio-coal”.
During the process, the biomass loses typically 20% of its mass (dry bone basis), while only 10% of the energy content in the biomass is lost. This energy (the volatiles) can be used as a heating fuel for the torrefaction process. After the biomass is torrefied it can be densified, usually into briquettes or pellets using conventional densification equipment, to further increase the density of the material and to improve its hydrophobic properties. With relation to brewing and food products, torrefication occurs when a cereal (barley, maize, oats, wheat, etc.) is cooked at high temperature to gelatinise the starch endosperm creating the expansion of the grain and creating a puffed apperance. The cereal can then be used whole or flaked. In brewing, the use of small quantities of torrefied wheat or barley in the mashing pocess aids in head retention and cling to the glass. Additionally, torrefied cereals are generally less expensive than equal amounts of malted products.
Added value of torrefied biomass
Torrefied and densified biomass has several advantages in different markets, which makes it a competitive option compared to conventional biomass (wood) pellets:
Higher energy density
Energy density of 18 - 20 GJ/m3 compared to 10 - 11 GJ/m3 driving a 40 - 50% reduction in transportation costs.
More homogeneous composition
Torrefied biomass can be produced from a wide variety of raw biomass feedstocks while yielding similar product properties. The main reason for this is that about all biomass are built from the same polymers (lignocellulose). In general (woody and herbaceous) biomass consists of three main polymeric structures: cellulose, hemicellulose and lignin. Together these are called lignocellulose. The chemical changes of these polymers during torrefaction are practically similar resulting in similar property changes.
Hydrophobic behavior
Torrefied biomass has hydrophobic properties, and when combined with densification make bulk storage in open air feasible.
Elimination of biological activity
All biological activity is eliminated reducing the risk of fire and stopping biological decomposition.
Improved grindability
Torrefaction of biomass leads to improved grindability of biomass. This leads to more efficient co-firing in existing coal fired power stations or entrained-flow gasification for the production of chemicals and transportation fuels.
Markets for torrefied biomass
Torrefied biomass has added value for different markets. Biomass in general provides a low-cost, low-risk route to lower CO2-emissions. When high volumes are needed, torrefaction can make biomass from distant sources price competitive.
Large scale co-firing in coal fired power plants
- Torrefied biomass results in lower handling costs;
- Torrefied biomass enables higher co-firing rates;
- Product can be delivered in a range of LHVs (20 – 25 GJ/ton) and sizes (briquette, pellet).
- Co-firing torrefied biomass with coal leads to reduction in net power plant emissions.
Steel production
- Fibrous biomass is very difficult to deploy in furnaces;
- To replace injection coal, biomass product needs to have LHV of more than 25 GJ/ton.
Residential/decentralized heating
- Relatively high percentage of transport on wheels as cost in supply chain makes biomass expensive. Increasing volumetric energy density does decrease costs;
- Limited storage space increases need for increased volumetric density;
- Moisture content important as moisture leads to smoke and smell.
Biomass-to-Liquids
- Torrefied biomass results in lower handling costs;
- Torrefied biomass serves as a ‘clean’ feedstock for production of transportation fuels (Fischer–Tropsch process), which saves considerably on production costs of such fuels.
Combustion
6:12 AM

Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame. Fuels of interest often include organic compounds (especially hydrocarbons) in the gas, liquid or solid phase.
In a complete combustion reaction, a compound reacts with an oxidizing element, such as oxygen or fluorine, and the products are compounds of each element in the fuel with the oxidizing element. For example:
- CH4 + 2O2 → CO2 + 2H2O + energy
- CH2S + 6F2 → CF4 + 2HF + SF6
A simple example can be seen in the combustion of hydrogen and oxygen, which is a commonly used reaction in rocket engines:
- 2H2 + O2 → 2H2O(g) + heat
The result is water vapor.
Complete combustion is almost impossible to achieve. In reality, as actual combustion reactions come to equilibrium, a wide variety of major and minor species will be present such as carbon monoxide and pure carbon (soot or ash). Additionally, any combustion in air, which is 78% nitrogen, will also create several forms of nitrogen oxides.
Biomass Gasification
5:13 AM

Gasification is a process that converts carbonaceous materials, such as coal, petroleum, biofuel, or biomass, into carbon monoxide and hydrogen by reacting the raw material, such as house waste, or compost at high temperatures with a controlled amount of oxygen and/or steam. The resulting gas mixture is called synthesis gas or syngas and is itself a fuel. Gasification is a method for extracting energy from many different types of organic materials.
The advantage of gasification is that using the syngas is potentially more efficient than direct combustion of the original fuel because it can be combusted at higher temperatures or even in fuel cells, so that the thermodynamic upper limit to the efficiency defined by Carnot's rule is higher or not applicable. Syngas may be burned directly in internal combustion engines, used to produce methanol and hydrogen, or converted via the Fischer-Tropsch process into synthetic fuel. Gasification can also begin with materials that are not otherwise useful fuels, such as biomass or organic waste. In addition, the high-temperature combustion refines out corrosive ash elements such as chloride and potassium, allowing clean gas production from otherwise problematic fuels.
Gasification of fossil fuels is currently widely used on industrial scales to generate electricity. However, almost any type of organic material can be used as the raw material for gasification, such as wood, biomass, or even plastic waste.
Gasification relies on chemical processes at elevated temperatures >700°C, which distinguishes it from biological processes such as anaerobic digestion that produce biogas.
Gasification Chemistry
In a gasifier, the carbonaceous material undergoes several different processes:
- The pyrolysis (or devolatilization) process occurs as the carbonaceous particle heats up. Volatiles are released and char is produced, resulting in up to 70% weight loss for coal. The process is dependent on the properties of the carbonaceous material and determines the structure and composition of the char, which will then undergo gasification reactions.
- The combustion process occurs as the volatile products and some of the char reacts with oxygen to form carbon dioxide and carbon monoxide, which provides heat for the subsequent gasification reactions. Letting C represent a carbon-containing organic compound, the basic reaction here is
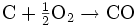
- The gasification process occurs as the char reacts with carbon dioxide and steam to produce carbon monoxide and hydrogen, via the reaction
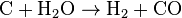
- In addition, the reversible gas phase water gas shift reaction reaches equilibrium very fast at the temperatures in a gasifier. This balances the concentrations of carbon monoxide, steam, carbon dioxide and hydrogen.

In essence, a limited amount of oxygen or air is introduced into the reactor to allow some of the organic material to be "burned" to produce carbon monoxide and energy, which drives a second reaction that converts further organic material to hydrogen and additional carbon dioxide. Further reactions occur when the formed carbon monoxide and residual water from the organic material react to form methane and excess carbon dioxide. This third reaction occurs more abundantly in reactors that increase the residence time of the reactive gases and organic materials, as well as heat and pressure. Catalysts are used in more sophisticated reactors to improve reaction rates, thus moving the system closer to the reaction equilibrium for a fixed residence time.
History
The gasification process was originally developed in the 1800s to produce town gas for lighting and cooking. Electricity and natural gas later replaced town gas for these applications, but the gasification process has been utilized for the production of synthetic chemicals and fuels since the 1920s.
Wood gas generators, called Gasogene or Gazogène, were used to power motor vehicles in Europe during World War II fuel shortages.
Gasification processes
Four types of gasifier are currently available for commercial use: counter-current fixed bed, co-current fixed bed, fluidized bed and entrained flow.
The counter-current fixed bed ("up draft") gasifier consists of a fixed bed of carbonaceous fuel (e.g. coal or biomass) through which the "gasification agent" (steam, oxygen and/or air) flows in counter-current configuration. The ash is either removed dry or as a slag. The slagging gasifiers have a lower ratio of steam to carbon, achieving temperatures higher than the ash fusion temperature. The nature of the gasifier means that the fuel must have high mechanical strength and must ideally be non-caking so that it will form a permeable bed, although recent developments have reduced these restrictions to some extent. The throughput for this type of gasifier is relatively low. Thermal efficiency is high as the gas exit temperatures are relatively low. However, this means that tar and methane production is significant at typical operation temperatures, so product gas must be extensively cleaned before use. The tar can be recycled to the reactor.
The co-current fixed bed ("down draft") gasifier is similar to the counter-current type, but the gasification agent gas flows in co-current configuration with the fuel (downwards, hence the name "down draft gasifier"). Heat needs to be added to the upper part of the bed, either by combusting small amounts of the fuel or from external heat sources. The produced gas leaves the gasifier at a high temperature, and most of this heat is often transferred to the gasification agent added in the top of the bed, resulting in an energy efficiency on level with the counter-current type. Since all tars must pass through a hot bed of char in this configuration, tar levels are much lower than the counter-current type.
In the fluidized bed reactor, the fuel is fluidized in oxygen and steam or air. The ash is removed dry or as heavy agglomerates that defluidize. The temperatures are relatively low in dry ash gasifiers, so the fuel must be highly reactive; low-grade coals are particularly suitable. The agglomerating gasifiers have slightly higher temperatures, and are suitable for higher rank coals. Fuel throughput is higher than for the fixed bed, but not as high as for the entrained flow gasifier. The conversion efficiency can be rather low due to elutriation of carbonaceous material. Recycle or subsequent combustion of solids can be used to increase conversion. Fluidized bed gasifiers are most useful for fuels that form highly corrosive ash that would damage the walls of slagging gasifiers. Biomass fuels generally contain high levels of corrosive ash.
In the entrained flow gasifier a dry pulverized solid, an atomized liquid fuel or a fuel slurry is gasified with oxygen (much less frequent: air) in co-current flow. The gasification reactions take place in a dense cloud of very fine particles. Most coals are suitable for this type of gasifier because of the high operating temperatures and because the coal particles are well separated from one another. The high temperatures and pressures also mean that a higher throughput can be achieved, however thermal efficiency is somewhat lower as the gas must be cooled before it can be cleaned with existing technology. The high temperatures also mean that tar and methane are not present in the product gas; however the oxygen requirement is higher than for the other types of gasifiers. All entrained flow gasifiers remove the major part of the ash as a slag as the operating temperature is well above the ash fusion temperature. A smaller fraction of the ash is produced either as a very fine dry fly ash or as a black colored fly ash slurry. Some fuels, in particular certain types of biomasses, can form slag that is corrosive for ceramic inner walls that serve to protect the gasifier outer wall. However some entrained bed type of gasifiers do not possess a ceramic inner wall but have an inner water or steam cooled wall covered with partially solidified slag. These types of gasifiers do not suffer from corrosive slags. Some fuels have ashes with very high ash fusion temperatures. In this case mostly limestone is mixed with the fuel prior to gasification. Addition of a little limestone will usually suffice for the lowering the fusion temperatures. The fuel particles must be much smaller than for other types of gasifiers. This means the fuel must be pulverized, which requires somewhat more energy than for the other types of gasifiers. By far the most energy consumption related to entrained bed gasification is not the milling of the fuel but the production of oxygen used for the gasification.
Current applications
In small business and building applications, where the wood source is sustainable, 250-1000 kWe and new zero carbon biomass gasification plants have been installed in Europe that produce tar free syngas from wood and burn it in a reciprocation engines connected to a generator with heat recovery. This type plant is often referred to as a wood biomass CHP unit but is a plant of seven different processes: biomass processing, fuel delivery, gasification, gas cleaning, waste disposal, electricity generation and heat recovery.
Industrial-scale gasification is currently mostly used to produce electricity from fossil fuels such as coal, where the syngas is burned in a gas turbine.
Gasification is also used industrially in the production of electricity, ammonia and liquid fuels (oil) using Integrated Gasification Combined Cycles (IGCC), with the possibility of producing methane and hydrogen for fuel cells. IGCC is also a more efficient method of CO2 capture as compared to conventional technologies. IGCC demonstration plants have been operating since the early 1970s and some of the plants constructed in the 1990s are now entering commercial service.
Gasification technologies have been developed in recent years that use plastic-rich waste as a feed.
Syngas can be used for heat production and for generation of mechanical and electrical power. Like other gaseous fuels, producer gas gives greater control over power levels when compared to solid fuels, leading to more efficient and cleaner operation.
Gasifiers offer a flexible option for thermal applications, as they can be retrofitted into existing gas fueled devices such as ovens, furnaces, boilers, etc., where syngas may replace fossil fuels. Heating values of syngas are generally around 4-10 MJ/m3.
Diesel engines can be operated on dual fuel mode using producer gas. Diesel substitution of over 80% at high loads and 70-80% under normal load variations can easily be achieved. Spark ignition engines and SOFC fuel cells can operate on 100% gasification gas. Mechanical energy from the engines may be used for e.g. driving water pumps for irrigation or for coupling with an alternator for electrical power generation.
Small-scale rural biomass gasifiers have been applied in India to a large extent, especially in the state of Tamil-Nadu in South India. Most of the applications are 9 kWe systems used for water pumping and street lighting operated by the local panchayat government. Although technically applicable the systems face political, financial and maintenance problems. Most of the systems are no longer running after 1–3 years.
While small scale gasifiers have existed for well over 100 years, there have been few sources to obtain a ready to use machine. Small scale devices are typically DIY projects. However, currently in the US several companies offer gasifiers to operate small engines.
Potential for renewable energy
In principle, gasification can proceed from just about any organic material, including biomass and plastic waste. The resulting syngas can be combusted. Alternatively, if the syngas is clean enough, it may be used for power production in gas engines, gas turbines or even fuel cells, or converted efficiently to dimethyl ether (DME) by methanol dehydration, methane via the Sabatier reaction, or diesel-like synthetic fuel via the Fischer-Tropsch process. In many gasification processes most of the inorganic components of the input material, such as metals and minerals, are retained in the ash. In some gasification processes (slagging gasification) this ash has the form of a glassy solid with low leaching properties, but the net power production in slagging gasification is low (sometimes negative) and costs are higher.
Regardless of the final fuel form, gasification itself and subsequent processing neither directly emits nor traps greenhouse gasses such as carbon dioxide. Power consumption in the gasification and syngas conversion processes may be significant though, and may indirectly cause CO2 emissions; in slagging and plasma gasification, the electricity consumption may even exceed any power production from the syngas. Combustion of syngas or derived fuels emits the exact same amount of carbon dioxide as would have been emitted from direct combustion of the initial fuel. Biomass gasification and combustion could play a significant role in a renewable energy economy, because biomass production removes the same amount of CO2 from the atmosphere as is emitted from gasification and combustion. While other biofuel technologies such as biogas and biodiesel are carbon neutral, gasification in principle may run on a wider variety of input materials and can be used to produce a wider variety of output fuels.
There is at present very little industrial scale biomass gasification being done. Examples of demonstration projects include those of the Renewable Energy Network Austria, including a plant using dual fluidized bed gasification that has supplied the town of Güssing with 2 MW of electricity and 4 MW of heat, generated from wood chips, since 2003.
Waste disposal
Several gasification processes for thermal treatment of waste are under development as an alternative to incineration.
Waste gasification has several principal advantages over incineration:
- The necessary extensive flue gas cleaning may be performed on the syngas instead of the much larger volume of flue gas after combustion.
- Electric power may be generated in engines and gas turbines, which are much cheaper and more efficient than the steam cycle used in incineration. Even fuel cells may potentially be used, but these have rather severe requirements regarding the purity of the gas.
- Chemical processing of the syngas may produce other synthetic fuels instead of electricity.
- Some gasification processes treat ash containing heavy metals at very high temperatures so that it is released in a glassy and chemically stable form.
 A major challenge for waste gasification technologies is to reach an acceptable (positive) gross electric efficiency. The high efficiency of converting syngas to electric power is counteracted by significant power consumption in the waste preprocessing, the consumption of large amounts of pure oxygen (which is often used as gasification agent), and gas cleaning. Another challenge becoming apparent when implementing the processes in real life is to obtain long service intervals in the plants, so that it is not necessary to close down the plant every few months for cleaning the reactor.
A major challenge for waste gasification technologies is to reach an acceptable (positive) gross electric efficiency. The high efficiency of converting syngas to electric power is counteracted by significant power consumption in the waste preprocessing, the consumption of large amounts of pure oxygen (which is often used as gasification agent), and gas cleaning. Another challenge becoming apparent when implementing the processes in real life is to obtain long service intervals in the plants, so that it is not necessary to close down the plant every few months for cleaning the reactor.
Several waste gasification processes have been proposed, but few have yet been built and tested, and only a handful have been implemented as plants processing real waste, and always in combination with fossil fuels.
One plant (in Chiba, Japan using the Thermoselect process) has been processing industrial waste since year 2000, but has not yet documented positive net energy production from the process.
Ze-gen is operating a waste gasification demonstration facility in New Bedford, Massachusetts. The facility was designed to demonstrate gasification of specific non-MSW waste streams using liquid metal gasification.
Trends in Incinerator Use
10:38 AM

The history of municipal solid waste (MSW) incineration is linked intimately to the history of landfills and other waste treatment technology. The merits of incineration are inevitably judged in relation to the alternatives available. Since the 1970s, recycling and other prevention measures have changed the context for such judgements. Since the 1990s alternative waste treatment technologies have been maturing and becoming viable.
Incineration is a key process in the treatment of hazardous wastes and clinical wastes. It is often imperative that medical waste be subjected to the high temperatures of incineration to destroy pathogens and toxic contamination it contains.
Incineration in North America
The first incinerator in the U.S. was built in 1885 on Governors Island in New York. In 1949, Robert C. Ross founded one of the first hazardous waste management companies in the U.S. He began Robert Ross Industrial Disposal because he saw an opportunity to meet the hazardous waste management needs of companies in northern Ohio. In 1958, the company built one of the first hazardous waste incinerators in the U.S. The first full-scale, municipally operated incineration facility in the U.S. was the Arnold O. Chantland Resource Recovery Plant, built in 1975 and located in Ames, Iowa. This plant is still in operation and produces refuse-derived fuel that is sent to local power plants for fuel.[51] The first commercially successful incineration plant in the U.S. was built in Saugus, Massachusetts in October 1975 by Wheelabrator Technologies, and is still in operation today.
There are several environmental or waste management corporations that transport ultimately to an incinerator or cement kiln treatment center. Currently (2009), there are three main businesses that incinerate waste: Clean Harbours, WTI-Heritage, and Ross Incineration Services. Clean Harbours has acquired many of the smaller, independently run facilties, accumulating 5–7 incinerators in the process across the U.S. WTI-Heritage has one incinerator, located in the southeastern corner of Ohio (across the Ohio River from West Virginia).
Several old generation incinerators have been closed; of the 186 MSW incinerators in 1990, only 89 remained by 2007, and of the 6200 medical waste incinerators in 1988, only 115 remained in 2003. No new incinerators were built between 1996 and 2007. The main reasons for lack of activity have been:
- Economics. With the increase in the number of large inexpensive regional landfills and, up until recently, the relatively low price of electricity, incinerators were not able to compete for the 'fuel', i.e., waste in the U.S.
- Tax policies. Tax credits for plants producing electricity from waste were rescinded in the U.S. between 1990 and 2004.
There has been renewed interest in incineration and other waste-to-energy technologies in the U.S. and Canada. In the U.S., incineration was granted qualification for renewable energy production tax credits in 2004. Projects to add capacity to existing plants are underway, and municipalities are once again evaluating the option of building incineration plants rather than continue landfilling municipal wastes. However, many of these projects have faced continued political opposition in spite of renewed arguments for the greenhouse gas benefits of incineration and improved air pollution control and ash recycling.
Incineration in Europe
In Europe, with the ban on landfilling untreated waste, scores of incinerators have been built in the last decade, with more under construction. Recently, a number of municipal governments have begun the process of contracting for the construction and operation of incinerators. In Europe, some of the electricity generated from waste is deemed to be from a 'Renewable Energy Source (RES)' and is thus eligible for tax credits if privately operated. Also, some incinerators in Europe are equipped with waste recovery, allowing the reuse of ferrous and non-ferrous materials found in landfills. A prominent example is the AEB Waste Fired Power Plant.
Incineration in the United Kingdom
The technology employed in the UK waste management industry has been greatly lagging behind that of Europe due to the wide availability of landfills. The Landfill Directive set down by the European Union led to the Government of the United Kingdom imposing waste legislation including the landfill tax and Landfill Allowance Trading Scheme. This legislation is designed to reduce the release of greenhouse gases produced by landfills through the use of alternative methods of waste treatment. It is the UK Government's position that incineration will play an increasingly large role in the treatment of municipal waste and supply of energy in the UK.
In the UK in 2008, plans for potential incinerator locations exists for approximately 100 sites. These have been interactively mapped by UK NGO's.
See the list of incinerators in the UK.
Small incinerator units
Small scale incinerators exist for special purposes. For example, the small scale incinerators are aimed for hygienically safe destruction of medical waste in developing countries. Small incinerators can be quickly deployed to remote areas where an outbreak has occurred to dispose of infected animals quickly and without the risk of cross contamination.Incineration Technology
12:56 AM

An incinerator is a furnace for burning waste. Modern incinerators include pollution mitigation equipment such as flue gas cleaning. There are various types of incinerator plant design: moving grate, fixed grate, rotary-kiln, and fluidised bed.
Burn pile
The burn pile, or burn pit is one of the simplest and earliest forms of waste disposal, essentially consisting of a mound of combustible materials piled on bare ground and set on fire. Indiscriminate piles of household waste are strongly discouraged and may be illegal in urban areas, but are permitted in certain rural situations such as clearing forested land for farming, where the stumps are uprooted and burned. Rural burn piles of organic yard waste are also sometimes permitted, though not asphalt shingles, plastics, or other petroleum products.
Burn piles can and have spread uncontrolled fires, for example if wind blows burning material off the pile into surrounding combustible grasses or onto buildings. As interior structures of the pile are consumed, the pile can shift and collapse, spreading the burn area. Even in a situation of no wind, small lightweight ignited embers can lift off the pile via convection, and waft through the air into grasses or onto buildings, igniting them.
Burn pits were used extensively by the U.S. military in Iraq and Afghanistan. Initial use was on an emergency basis but use continued for extended periods of time, sometimes years. There have be complaints by military personnel and veterans that toxic chemicals from the burn pits resulted in respiratory problems.
Burn barrel
The burn barrel is a somewhat more controlled form of private waste incineration, containing the burning material inside a metal barrel, with a metal grating over the exhaust. The barrel prevents the spread of burning material in windy conditions, and as the combustibles are reduced they can only settle down into the barrel. The exhaust grating helps to prevent the spread of burning embers. Typically steel 55-gallon drums are used as burn barrels, with air vent holes cut or drilled around the base for air intake. Over time the very high heat of incineration causes the metal to oxidize and rust, and eventually the barrel itself is consumed by the heat and must be replaced.
Private burning of dry cellulosic/paper products is generally clean-burning, producing no visible smoke, but the large amount of plastics in household waste can cause private burning to create a public nuisance and health hazard, generating acrid odors and fumes that make eyes burn and water. The temperatures in a burn barrel are not regulated, and usually do not reach high enough or for enough time to completely break down chemicals such as dioxin in plastics and other waste chemicals. Therefore plastics and other petroleum products must be separated and sent to commercial waste disposal facilities.
Private rural incineration is typically only permitted so long as it is not a nuisance to others, does not pose a risk of fire such as in dry conditions, and the fire is clean-burning, producing no visible smoke. People intending to burn waste may be required to contact a state agency in advance to check current fire risk and conditions, and to alert officials of the controlled fire that will occur.
Moving grate
The typical incineration plant for municipal solid waste is a moving grate incinerator. The moving grate enables the movement of waste through the combustion chamber to be optimised to allow a more efficient and complete combustion. A single moving grate boiler can handle up to 35 metric tons (39 short tons) of waste per hour, and can operate 8,000 hours per year with only one scheduled stop for inspection and maintenance of about one month's duration. Moving grate incinerators are sometimes referred to as Municipal Solid Waste Incinerators (MSWIs).
The waste is introduced by a waste crane through the "throat" at one end of the grate, from where it moves down over the descending grate to the ash pit in the other end. Here the ash is removed through a water lock.
Part of the combustion air (primary combustion air) is supplied through the grate from below. This air flow also has the purpose of cooling the grate itself. Cooling is important for the mechanical strength of the grate, and many moving grates are also water cooled internally.
Secondary combustion air is supplied into the boiler at high speed through nozzles over the grate. It facilitates complete combustion of the flue gases by introducing turbulence for better mixing and by ensuring a surplus of oxygen. In multiple/stepped hearth incinerators, the secondary combustion air is introduced in a separate chamber downstream the primary combustion chamber.
According to the European Waste Incineration Directive, incineration plants must be designed to ensure that the flue gases reach a temperature of at least 850 °C (1,560 °F) for 2 seconds in order to ensure proper breakdown of toxic organic substances. In order to comply with this at all times, it is required to install backup auxiliary burners (often fueled by oil), which are fired into the boiler in case the heating value of the waste becomes too low to reach this temperature alone.
The flue gases are then cooled in the superheaters, where the heat is transferred to steam, heating the steam to typically 400 °C (752 °F) at a pressure of 40 bars (580 psi) for the electricity generation in the turbine. At this point, the flue gas has a temperature of around 200 °C (392 °F), and is passed to the flue gas cleaning system.
In Scandinavia scheduled maintenance is always performed during summer, where the demand for district heating is low. Often incineration plants consist of several separate 'boiler lines' (boilers and flue gas treatment plants), so that waste can continue to be received at one boiler line while the others are subject to revision.
Fixed grate
The older and simpler kind of incinerator was a brick-lined cell with a fixed metal grate over a lower ash pit, with one opening in the top or side for loading and another opening in the side for removing incombustible solids called clinkers. Many small incinerators formerly found in apartment houses have now been replaced by waste compactors.
Rotary-kiln
The rotary-kiln incinerator is used by municipalities and by large industrial plants. This design of incinerator has 2 chambers: a primary chamber and secondary chamber. The primary chamber in a rotary kiln incinerator consist of an inclined refractory lined cylindrical tube. Movement of the cylinder on its axis facilitates movement of waste. In the primary chamber, there is conversion of solid fraction to gases, through volatilization, destructive distillation and partial combustion reactions. The secondary chamber is necessary to complete gas phase combustion reactions.
The clinkers spill out at the end of the cylinder. A tall flue gas stack, fan, or steam jet supplies the needed draft. Ash drops through the grate, but many particles are carried along with the hot gases. The particles and any combustible gases may be combusted in an "afterburner".
Fluidized bed
A strong airflow is forced through a sandbed. The air seeps through the sand until a point is reached where the sand particles separate to let the air through and mixing and churning occurs, thus a fluidised bed is created and fuel and waste can now be introduced.
The sand with the pre-treated waste and/or fuel is kept suspended on pumped air currents and takes on a fluid-like character. The bed is thereby violently mixed and agitated keeping small inert particles and air in a fluid-like state. This allows all of the mass of waste, fuel and sand to be fully circulated through the furnace.
Specialized incineration
Furniture factory sawdust incinerators need much attention as these have to handle resin powder and many flammable substances. Controlled combustion, burn back prevention systems are essential as dust when suspended resembles the fire catch phenomenon of any liquid petroleum gas.
Use of heat
The heat produced by an incinerator can be used to generate steam which may then be used to drive a turbine in order to produce electricity. The typical amount of net energy that can be produced per tonne municipal waste is about 2/3 MWh of electricity and 2 MWh of district heating. Thus, incinerating about 600 metric tons (660 short tons) per day of waste will produce about 400 MWh of electrical energy per day (17 MW of electrical power continuously for 24 hours) and 1200 MWh of district heating energy each day.
Pollution
Incineration has a number of outputs such as the ash and the emission to the atmosphere of flue gas. Before the flue gas cleaning system, the flue gases may contain significant amounts of particulate matter, heavy metals, dioxins, furans, sulfur dioxide, and hydrochloric acid.
In a study from 1994, Delaware Solid Waste Authority found that, for same amount of produced energy, incineration plants emitted fewer particles, hydrocarbons and less SO2, HCl, CO and NOx than coal-fired power plants, but more than natural gas fired power plants. According to Germany's Ministry of the Environment, waste incinerators reduce the amount of some atmospheric pollutants by substituting power produced by coal-fired plants with power from waste-fired plants.
Gaseous emissions
Dioxin and furans
The most publicized concerns from environmentalists about the incineration of municipal solid wastes (MSW) involve the fear that it produces significant amounts of dioxin and furan emissions. Dioxins and furans are considered by many to be serious health hazards.
In 2005, The Ministry of the Environment of Germany, where there were 66 incinerators at that time, estimated that "...whereas in 1990 one third of all dioxin emissions in Germany came from incineration plants, for the year 2000 the figure was less than 1 %. Chimneys and tiled stoves in private households alone discharge approximately 20 times more dioxin into the environment than incineration plants."
According to the United States Environmental Protection Agency, incineration plants are no longer significant sources of dioxins and furans. In 1987, before the governmental regulations required the use of emission controls, there was a total of 10,000 grams (350 oz) of dioxin emissions from US incinerators. Today, the total emissions from the 87 plants are only 10 grams (0.35 oz) yearly, a reduction of 99.9 %.
Backyard barrel burning of household and garden wastes, still allowed in some rural areas, generates 580 grams (20 oz) of dioxins yearly. Studies conducted by the US-EPA demonstrate that the emissions from just one family using a burn barrel produces more emissions than an incineration plant disposing of 200 metric tons (220 short tons) of waste per day.
Dioxin cracking methods and limitations
Generally, the breakdown of dioxin requires exposure of the molecular ring to a sufficiently high temperature so as to trigger thermal breakdown of the strong molecular bonds holding it together. Small pieces of fly ash may be somewhat thick, and too brief an exposure to high temperature may only degrade dioxin on the surface of the ash. For a large volume air chamber, too brief an exposure may also result in only some of the exhaust gases reaching the full breakdown temperature. For this reason there is also a time element to the temperature exposure to ensure heating completely through the thickness of the fly ash and the volume of waste gases.
There are trade-offs between increasing either the temperature or exposure time. Generally where the molecular breakdown temperature is higher, the exposure time for heating can be shorter, but excessively high temperatures can also cause wear and damage to other parts of the incineration equipment. Likewise the breakdown temperature can be lowered to some degree but then the exhaust gases would require a greater lingering period of perhaps several minutes, which would require large/long treatment chambers that take up a great deal of treatment plant space.
A side effect of breaking the strong molecular bonds of dioxin is the potential for breaking the bonds of nitrogen gas (N2) and oxygen gas (O2) in the supply air. As the exhaust flow cools, these highly reactive detached atoms spontaneously reform bonds into reactive oxides such as NOx in the flue gas, which can result in smog formation and acid rain if they were released directly into the local environment. These reactive oxides must be further neutralized with selective catalytic reduction (SCR) or selective non-catalytic reduction.
Dioxin cracking in practice
The temperatures needed to break down dioxin are typically not reached when burning of plastics outdoors in a burn barrel or garbage pit, causing high dioxin emissions as mentioned above. While plastic does usually burn in an open-air fire, the dioxins remain after combustion and either float off into the atmosphere, or may remain in the ash where it can be leached down into groundwater when rain falls on the ash pile.
Modern municipal incinerator designs include a high temperature zone, where the flue gas is ensured to sustain a temperature above 850 °C (1,560 °F) for at least 2 seconds before it is cooled down. They are equipped with auxiliary heaters to ensure this at all times. These are often fueled by oil, and normally only active for a very small fraction of the time.
For very small municipal incinerators, the required temperature for thermal breakdown of dioxin may be reached using a high-temperature electrical heating element, plus a selective catalytic reduction stage.
CO2
As for other complete combustion processes, nearly all of the carbon content in the waste is emitted as CO2 to the atmosphere. MSW contains approximately the same mass fraction of carbon as CO2 itself (27%), so incineration of 1 ton of MSW produces approximately 1 ton of CO2.
If the waste was landfilled, 1 ton of MSW would produce approximately 62 cubic metres (2,200 cu ft) methane via the anaerobic decomposition of the biodegradable part of the waste. This much methane has more than twice the global warming potential than the 1 ton of CO2, which would have been produced by incineration. In some countries, large amounts of landfill gas are collected, but still the global warming potential of the landfill gas emitted to atmosphere in the US in 1999 was approximately 32 % higher than the amount of CO2 that would have been emitted by incineration.
In addition, nearly all biodegradable waste has biological origin. This material has been formed by plants using atmospheric CO2 typically within the last growing season. If these plants are regrown the CO2 emitted from their combustion will be taken out from the atmosphere once more.
Such considerations are the main reason why several countries administrate incineration of the biodegradable part of waste as renewable energy. The rest – mainly plastics and other oil and gas derived products – is generally treated as non-renewables.
Different results for the CO2 footprint of incineration can be reached with different assumptions. Local conditions (such as limited local district heating demand, no fossil fuel generated electricity to replace or high levels of aluminum in the waste stream) can decrease the CO2 benefits of incineration. The methodology and other assumptions may also influence the results significantly. For example the methane emissions from landfills occurring at a later date may be neglected or given less weight, or biodegradable waste may not be considered CO2 neutral. A recent study by Eunomia Research and Consulting on potential waste treatment technologies in London demonstrated that by applying several of these (according to the authors) unusual assumptions the average existing incineration plants performed poorly for CO2 balance compared to the theoretical potential of other emerging waste treatment technologies.
Other emissions
Other gaseous emissions in the flue gas from incinerator furnaces include sulfur dioxide, hydrochloric acid, heavy metals and fine particles.
The steam content in the flue may produce visible fume from the stack, which can be perceived as a visual pollution. It may be avoided by decreasing the steam content by flue gas condensation and reheating, or by increasing the flue gas exit temperature well above its dew point. Flue gas condensation allows the latent heat of vaporization of the water to be recovered, subsequently increasing the thermal efficiency of the plant.
Flue gas cleaning
The quantity of pollutants in the flue gas from incineration plants is reduced by several processes.
Particulate is collected by particle filtration, most often electrostatic precipitators (ESP) and/or baghouse filters. The latter are generally very efficient for collecting fine particles. In an investigation by the Ministry of the Environment of Denmark in 2006, the average particulate emissions per energy content of incinerated waste from 16 Danish incinerators were below 2.02 g/GJ (grams per energy content of the incinerated waste). Detailed measurements of fine particles with sizes below 2.5 micrometres (PM2.5) were performed on three of the incinerators: One incinerator equipped with an ESP for particle filtration emitted 5.3 g/GJ fine particles, while two incinerators equipped with baghouse filters emitted 0.002 and 0.013 g/GJ PM2.5. For ultra fine particles (PM1.0), the numbers were 4.889 g/GJ PM1.0 from the ESP plant, while emissions of 0.000 and 0.008 g/GJ PM1.0 were measured from the plants equipped with baghouse filters.
Acid gas scrubbers are used to remove hydrochloric acid, nitric acid, hydrofluoric acid, mercury, lead and other heavy metals. Basic scrubbers remove sulfur dioxide, forming gypsum by reaction with lime.
Waste water from scrubbers must subsequently pass through a waste water treatment plant.
Sulfur dioxide may also be removed by dry desulfurisation by injection limestone slurry into the flue gas before the particle filtration.
NOx is either reduced by catalytic reduction with ammonia in a catalytic converter (selective catalytic reduction, SCR) or by a high temperature reaction with ammonia in the furnace (selective non-catalytic reduction, SNCR). Urea may be substituted for ammonia as the reducing reagent but must be supplied earlier in the process so that it can hydrolyze into ammonia. Substitution of urea can reduce costs and potential hazards associated with storage of anhydrous ammonia.
Heavy metals are often adsorbed on injected active carbon powder, which is collected by the particle filtration.
Solid outputs
Incineration produces fly ash and bottom ash just as is the case when coal is combusted. The total amount of ash produced by municipal solid waste incineration ranges from 4-10 % by volume and 15-20 % by weight of the original quantity of waste, and the fly ash amounts to about 10-20 % of the total ash. The fly ash, by far, constitutes more of a potential health hazard than does the bottom ash because the fly ash often contain high concentrations of heavy metals such as lead, cadmium, copper and zinc as well as small amounts of dioxins and furans. The bottom ash seldom contain significant levels of heavy metals. In testing over the past decade, no ash from an incineration plant in the USA has ever been determined to be a hazardous waste. At present although some historic samples tested by the incinerator operators' group would meet the being ecotoxic criteria at present the EA say "we have agreed" to regard incinerator bottom ash as "non-hazardous" until the testing programme is complete.
Other pollution issues
Odor pollution can be a problem with old-style incinerators, but odors and dust are extremely well controlled in newer incineration plants. They receive and store the waste in an enclosed area with a negative pressure with the airflow being routed through the boiler which prevents unpleasant odors from escaping into the atmosphere. However, not all plants are implemented this way, resulting in inconveniences in the locality.
An issue that affects community relationships is the increased road traffic of waste collection vehicles to transport municipal waste to the incinerator. Due to this reason, most incinerators are located in industrial areas. This problem can be can avoided to an extent through the transport of waste by rail from transfer stations.
Incineration
2:41 PM

Incineration is a waste treatment process that involves the combustion of organic substances contained in waste materials. Incineration and other high temperature waste treatment systems are described as "thermal treatment". Incineration of waste materials converts the waste into ash, flue gas, and heat. The ash is mostly formed by the inorganic constituents of the waste, and may take the form of solid lumps or particulates carried by the flue gas. The flue gases must be cleaned of gaseous and particulate pollutants before they are dispersed into the atmosphere. In some cases, the heat generated by incineration can be used to generate electric power.
Incineration with energy recovery is one of several waste-to-energy (WtE) technologies such as gasification, Plasma arc gasification, pyrolysis and anaerobic digestion. Incineration may also be implemented without energy and materials recovery.
In several countries, there are still concerns from experts and local communities about the environmental impact of incinerators.
In some countries, incinerators built just a few decades ago often did not include a materials separation to remove hazardous, bulky or recyclable materials before combustion. These facilities tended to risk the health of the plant workers and the local environment due to inadequate levels of gas cleaning and combustion process control. Most of these facilities did not generate electricity.
Incinerators reduce the mass of the original waste by 80–85 % and the volume (already compressed somewhat in garbage trucks) by 95-96 %, depending on composition and degree of recovery of materials such as metals from the ash for recycling. This means that while incineration does not completely replace landfilling, it significantly reduces the necessary volume for disposal. Garbage trucks often reduce the volume of waste in a built-in compressor before delivery to the incinerator. Alternatively, at landfills, the volume of the uncompressed garbage can be reduced by approximately 70% by using a stationary steel compressor, albeit with a significant energy cost. In many countries, simpler waste compaction is a common practice for compaction at landfills.
Incineration has particularly strong benefits for the treatment of certain waste types in niche areas such as clinical wastes and certain hazardous wastes where pathogens and toxins can be destroyed by high temperatures. Examples include chemical multi-product plants with diverse toxic or very toxic wastewater streams, which cannot be routed to a conventional wastewater treatment plant.
Waste combustion is particularly popular in countries such as Japan where land is a scarce resource. Denmark and Sweden have been leaders in using the energy generated from incineration for more than a century, in localised combined heat and power facilities supporting district heating schemes. In 2005, waste incineration produced 4.8 % of the electricity consumption and 13.7 % of the total domestic heat consumption in Denmark. A number of other European countries rely heavily on incineration for handling municipal waste, in particular Luxembourg, the Netherlands, Germany and France.