Biomass Gasification
5:13 AM

Gasification is a process that converts carbonaceous materials, such as coal, petroleum, biofuel, or biomass, into carbon monoxide and hydrogen by reacting the raw material, such as house waste, or compost at high temperatures with a controlled amount of oxygen and/or steam. The resulting gas mixture is called synthesis gas or syngas and is itself a fuel. Gasification is a method for extracting energy from many different types of organic materials.
The advantage of gasification is that using the syngas is potentially more efficient than direct combustion of the original fuel because it can be combusted at higher temperatures or even in fuel cells, so that the thermodynamic upper limit to the efficiency defined by Carnot's rule is higher or not applicable. Syngas may be burned directly in internal combustion engines, used to produce methanol and hydrogen, or converted via the Fischer-Tropsch process into synthetic fuel. Gasification can also begin with materials that are not otherwise useful fuels, such as biomass or organic waste. In addition, the high-temperature combustion refines out corrosive ash elements such as chloride and potassium, allowing clean gas production from otherwise problematic fuels.
Gasification of fossil fuels is currently widely used on industrial scales to generate electricity. However, almost any type of organic material can be used as the raw material for gasification, such as wood, biomass, or even plastic waste.
Gasification relies on chemical processes at elevated temperatures >700°C, which distinguishes it from biological processes such as anaerobic digestion that produce biogas.
Gasification Chemistry
In a gasifier, the carbonaceous material undergoes several different processes:
- The pyrolysis (or devolatilization) process occurs as the carbonaceous particle heats up. Volatiles are released and char is produced, resulting in up to 70% weight loss for coal. The process is dependent on the properties of the carbonaceous material and determines the structure and composition of the char, which will then undergo gasification reactions.
- The combustion process occurs as the volatile products and some of the char reacts with oxygen to form carbon dioxide and carbon monoxide, which provides heat for the subsequent gasification reactions. Letting C represent a carbon-containing organic compound, the basic reaction here is
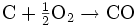
- The gasification process occurs as the char reacts with carbon dioxide and steam to produce carbon monoxide and hydrogen, via the reaction
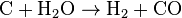
- In addition, the reversible gas phase water gas shift reaction reaches equilibrium very fast at the temperatures in a gasifier. This balances the concentrations of carbon monoxide, steam, carbon dioxide and hydrogen.

In essence, a limited amount of oxygen or air is introduced into the reactor to allow some of the organic material to be "burned" to produce carbon monoxide and energy, which drives a second reaction that converts further organic material to hydrogen and additional carbon dioxide. Further reactions occur when the formed carbon monoxide and residual water from the organic material react to form methane and excess carbon dioxide. This third reaction occurs more abundantly in reactors that increase the residence time of the reactive gases and organic materials, as well as heat and pressure. Catalysts are used in more sophisticated reactors to improve reaction rates, thus moving the system closer to the reaction equilibrium for a fixed residence time.
History
The gasification process was originally developed in the 1800s to produce town gas for lighting and cooking. Electricity and natural gas later replaced town gas for these applications, but the gasification process has been utilized for the production of synthetic chemicals and fuels since the 1920s.
Wood gas generators, called Gasogene or Gazogène, were used to power motor vehicles in Europe during World War II fuel shortages.
Gasification processes
Four types of gasifier are currently available for commercial use: counter-current fixed bed, co-current fixed bed, fluidized bed and entrained flow.
The counter-current fixed bed ("up draft") gasifier consists of a fixed bed of carbonaceous fuel (e.g. coal or biomass) through which the "gasification agent" (steam, oxygen and/or air) flows in counter-current configuration. The ash is either removed dry or as a slag. The slagging gasifiers have a lower ratio of steam to carbon, achieving temperatures higher than the ash fusion temperature. The nature of the gasifier means that the fuel must have high mechanical strength and must ideally be non-caking so that it will form a permeable bed, although recent developments have reduced these restrictions to some extent. The throughput for this type of gasifier is relatively low. Thermal efficiency is high as the gas exit temperatures are relatively low. However, this means that tar and methane production is significant at typical operation temperatures, so product gas must be extensively cleaned before use. The tar can be recycled to the reactor.
The co-current fixed bed ("down draft") gasifier is similar to the counter-current type, but the gasification agent gas flows in co-current configuration with the fuel (downwards, hence the name "down draft gasifier"). Heat needs to be added to the upper part of the bed, either by combusting small amounts of the fuel or from external heat sources. The produced gas leaves the gasifier at a high temperature, and most of this heat is often transferred to the gasification agent added in the top of the bed, resulting in an energy efficiency on level with the counter-current type. Since all tars must pass through a hot bed of char in this configuration, tar levels are much lower than the counter-current type.
In the fluidized bed reactor, the fuel is fluidized in oxygen and steam or air. The ash is removed dry or as heavy agglomerates that defluidize. The temperatures are relatively low in dry ash gasifiers, so the fuel must be highly reactive; low-grade coals are particularly suitable. The agglomerating gasifiers have slightly higher temperatures, and are suitable for higher rank coals. Fuel throughput is higher than for the fixed bed, but not as high as for the entrained flow gasifier. The conversion efficiency can be rather low due to elutriation of carbonaceous material. Recycle or subsequent combustion of solids can be used to increase conversion. Fluidized bed gasifiers are most useful for fuels that form highly corrosive ash that would damage the walls of slagging gasifiers. Biomass fuels generally contain high levels of corrosive ash.
In the entrained flow gasifier a dry pulverized solid, an atomized liquid fuel or a fuel slurry is gasified with oxygen (much less frequent: air) in co-current flow. The gasification reactions take place in a dense cloud of very fine particles. Most coals are suitable for this type of gasifier because of the high operating temperatures and because the coal particles are well separated from one another. The high temperatures and pressures also mean that a higher throughput can be achieved, however thermal efficiency is somewhat lower as the gas must be cooled before it can be cleaned with existing technology. The high temperatures also mean that tar and methane are not present in the product gas; however the oxygen requirement is higher than for the other types of gasifiers. All entrained flow gasifiers remove the major part of the ash as a slag as the operating temperature is well above the ash fusion temperature. A smaller fraction of the ash is produced either as a very fine dry fly ash or as a black colored fly ash slurry. Some fuels, in particular certain types of biomasses, can form slag that is corrosive for ceramic inner walls that serve to protect the gasifier outer wall. However some entrained bed type of gasifiers do not possess a ceramic inner wall but have an inner water or steam cooled wall covered with partially solidified slag. These types of gasifiers do not suffer from corrosive slags. Some fuels have ashes with very high ash fusion temperatures. In this case mostly limestone is mixed with the fuel prior to gasification. Addition of a little limestone will usually suffice for the lowering the fusion temperatures. The fuel particles must be much smaller than for other types of gasifiers. This means the fuel must be pulverized, which requires somewhat more energy than for the other types of gasifiers. By far the most energy consumption related to entrained bed gasification is not the milling of the fuel but the production of oxygen used for the gasification.
Current applications
In small business and building applications, where the wood source is sustainable, 250-1000 kWe and new zero carbon biomass gasification plants have been installed in Europe that produce tar free syngas from wood and burn it in a reciprocation engines connected to a generator with heat recovery. This type plant is often referred to as a wood biomass CHP unit but is a plant of seven different processes: biomass processing, fuel delivery, gasification, gas cleaning, waste disposal, electricity generation and heat recovery.
Industrial-scale gasification is currently mostly used to produce electricity from fossil fuels such as coal, where the syngas is burned in a gas turbine.
Gasification is also used industrially in the production of electricity, ammonia and liquid fuels (oil) using Integrated Gasification Combined Cycles (IGCC), with the possibility of producing methane and hydrogen for fuel cells. IGCC is also a more efficient method of CO2 capture as compared to conventional technologies. IGCC demonstration plants have been operating since the early 1970s and some of the plants constructed in the 1990s are now entering commercial service.
Gasification technologies have been developed in recent years that use plastic-rich waste as a feed.
Syngas can be used for heat production and for generation of mechanical and electrical power. Like other gaseous fuels, producer gas gives greater control over power levels when compared to solid fuels, leading to more efficient and cleaner operation.
Gasifiers offer a flexible option for thermal applications, as they can be retrofitted into existing gas fueled devices such as ovens, furnaces, boilers, etc., where syngas may replace fossil fuels. Heating values of syngas are generally around 4-10 MJ/m3.
Diesel engines can be operated on dual fuel mode using producer gas. Diesel substitution of over 80% at high loads and 70-80% under normal load variations can easily be achieved. Spark ignition engines and SOFC fuel cells can operate on 100% gasification gas. Mechanical energy from the engines may be used for e.g. driving water pumps for irrigation or for coupling with an alternator for electrical power generation.
Small-scale rural biomass gasifiers have been applied in India to a large extent, especially in the state of Tamil-Nadu in South India. Most of the applications are 9 kWe systems used for water pumping and street lighting operated by the local panchayat government. Although technically applicable the systems face political, financial and maintenance problems. Most of the systems are no longer running after 1–3 years.
While small scale gasifiers have existed for well over 100 years, there have been few sources to obtain a ready to use machine. Small scale devices are typically DIY projects. However, currently in the US several companies offer gasifiers to operate small engines.
Potential for renewable energy
In principle, gasification can proceed from just about any organic material, including biomass and plastic waste. The resulting syngas can be combusted. Alternatively, if the syngas is clean enough, it may be used for power production in gas engines, gas turbines or even fuel cells, or converted efficiently to dimethyl ether (DME) by methanol dehydration, methane via the Sabatier reaction, or diesel-like synthetic fuel via the Fischer-Tropsch process. In many gasification processes most of the inorganic components of the input material, such as metals and minerals, are retained in the ash. In some gasification processes (slagging gasification) this ash has the form of a glassy solid with low leaching properties, but the net power production in slagging gasification is low (sometimes negative) and costs are higher.
Regardless of the final fuel form, gasification itself and subsequent processing neither directly emits nor traps greenhouse gasses such as carbon dioxide. Power consumption in the gasification and syngas conversion processes may be significant though, and may indirectly cause CO2 emissions; in slagging and plasma gasification, the electricity consumption may even exceed any power production from the syngas. Combustion of syngas or derived fuels emits the exact same amount of carbon dioxide as would have been emitted from direct combustion of the initial fuel. Biomass gasification and combustion could play a significant role in a renewable energy economy, because biomass production removes the same amount of CO2 from the atmosphere as is emitted from gasification and combustion. While other biofuel technologies such as biogas and biodiesel are carbon neutral, gasification in principle may run on a wider variety of input materials and can be used to produce a wider variety of output fuels.
There is at present very little industrial scale biomass gasification being done. Examples of demonstration projects include those of the Renewable Energy Network Austria, including a plant using dual fluidized bed gasification that has supplied the town of Güssing with 2 MW of electricity and 4 MW of heat, generated from wood chips, since 2003.
Waste disposal
Several gasification processes for thermal treatment of waste are under development as an alternative to incineration.
Waste gasification has several principal advantages over incineration:
- The necessary extensive flue gas cleaning may be performed on the syngas instead of the much larger volume of flue gas after combustion.
- Electric power may be generated in engines and gas turbines, which are much cheaper and more efficient than the steam cycle used in incineration. Even fuel cells may potentially be used, but these have rather severe requirements regarding the purity of the gas.
- Chemical processing of the syngas may produce other synthetic fuels instead of electricity.
- Some gasification processes treat ash containing heavy metals at very high temperatures so that it is released in a glassy and chemically stable form.
 A major challenge for waste gasification technologies is to reach an acceptable (positive) gross electric efficiency. The high efficiency of converting syngas to electric power is counteracted by significant power consumption in the waste preprocessing, the consumption of large amounts of pure oxygen (which is often used as gasification agent), and gas cleaning. Another challenge becoming apparent when implementing the processes in real life is to obtain long service intervals in the plants, so that it is not necessary to close down the plant every few months for cleaning the reactor.
A major challenge for waste gasification technologies is to reach an acceptable (positive) gross electric efficiency. The high efficiency of converting syngas to electric power is counteracted by significant power consumption in the waste preprocessing, the consumption of large amounts of pure oxygen (which is often used as gasification agent), and gas cleaning. Another challenge becoming apparent when implementing the processes in real life is to obtain long service intervals in the plants, so that it is not necessary to close down the plant every few months for cleaning the reactor.
Several waste gasification processes have been proposed, but few have yet been built and tested, and only a handful have been implemented as plants processing real waste, and always in combination with fossil fuels.
One plant (in Chiba, Japan using the Thermoselect process) has been processing industrial waste since year 2000, but has not yet documented positive net energy production from the process.
Ze-gen is operating a waste gasification demonstration facility in New Bedford, Massachusetts. The facility was designed to demonstrate gasification of specific non-MSW waste streams using liquid metal gasification.